Summary
Fluorosilicone medical release liners help improve the reliability, durability, and ease of use of medical devices, ensuring that the adhesive surfaces remain protected until they are ready for application. This is vital in medical applications where precision and sterility are crucial factors.
What Are Medical Release Liners?
Medical release liners must meet several criteria to match medical regulatory standards while remaining easy to use for consumers.
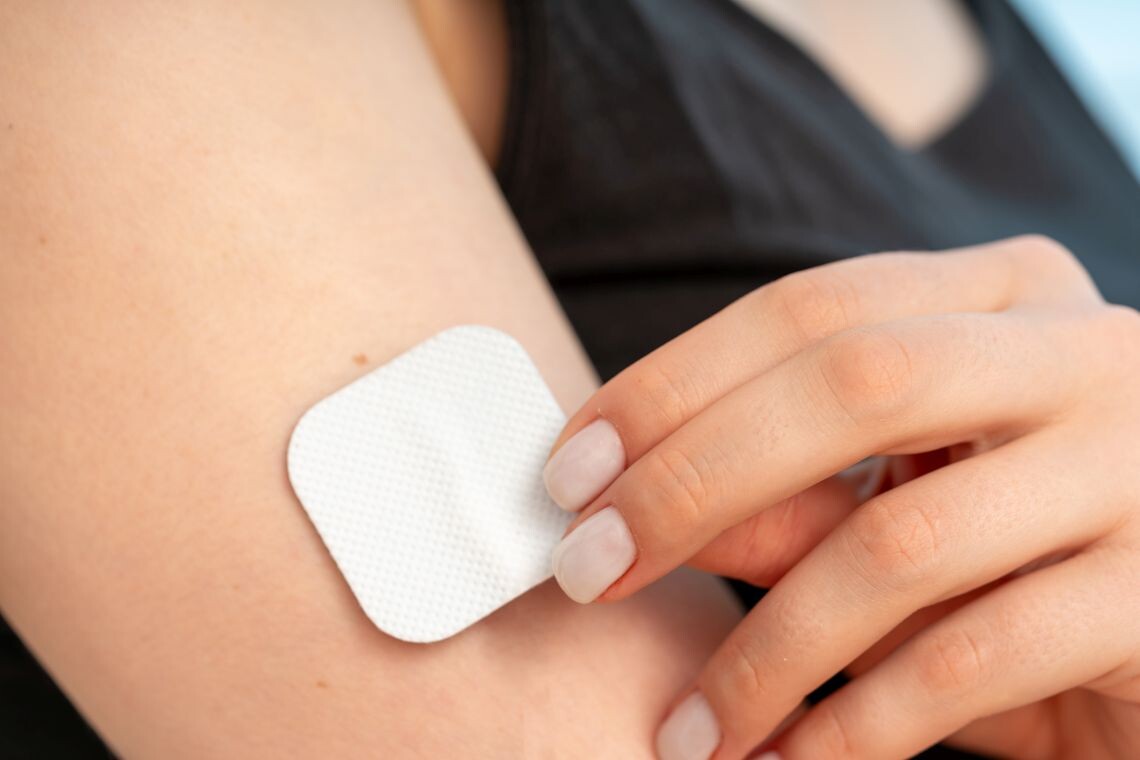
Medical release liners are thin sheets or films used to protect adhesive surfaces on medical devices such as wound dressings, transdermal patches, or ostomy products. The primary purpose of the release liner is to protect the adhesive before use and ensure the adhesive does not prematurely stick to unintended surfaces. When the user is ready to apply the medical device, they can easily peel off the release liner without damaging the adhesive or the device itself.
When an application needs release coated films, these products are tailor-engineered to meet exact technical needs and provide reassurance in terms of compliance with mandatory federal regulations.
Medical Applications
Among the other medical applications prominent in the use of release liners are diagnostic test strips, transdermal drug delivery systems, surgical wound care, medical sensors, cosmetic patches, and process carrier liners.
The effectiveness of the liners comes down to the properties of the materials that make them up, and one such material is fluorosilicone. Fluorosilicone is a specialized type of silicone material that has been engineered to have enhanced properties, such as resistance to chemicals and extreme temperatures.
Benefits of Fluorosilicone Release Liners
Non-stick properties: Fluorosilicone has a low coefficient of friction, so has excellent non-stick properties. It prevents the adhesive from sticking to the release liner too strongly, making it easier for users to peel off the liner when applying the medical device.
Chemical resistance: Fluorosilicone is highly resistant to chemicals and solvents. This characteristic is crucial for medical devices that encounter various fluids, ointments, or cleaning agents. The fluorosilicone coating ensures that the release liner remains intact and does not degrade when exposed to these substances.
Temperature resistance: Medical devices and products often need to withstand a range of temperatures, including during transportation, storage, and usage. Fluorosilicone coatings can withstand a broader range of temperatures than regular silicone, ensuring that the release liners remain effective and functional even in extreme conditions.
Moisture resistance: Fluorosilicone is highly resistant to moisture and humidity, which is important in maintaining the performance of medical adhesives. The coating prevents the release liner from absorbing moisture, which could compromise the adhesive.
To learn more about how CoreTech flourosilicone release liners contact one of our experts today.